The world is starting to win the war against tuberculosis, but drug-resistant forms pose a new threat.
A chest X-ray from a patient with tuberculosis (TB) in Lira, Uganda. Uganda is one of 22 countries accounting for roughly 80% of new TB cases each year.
J. MATTHEWS/PANOS
If there was any doubt that tuberculosis (TB) was fighting back, it was dispelled in 2005, at the Church of Scotland Hospital in the village of Tugela Ferry, South Africa. Doctors at the hospital, in a rough, remote corner of KwaZulu-Natal province, were hardened to people dying from gunshots and AIDS. But even they were puzzled and frightened when patients with HIV who were responding well to antiretroviral drugs began dying — rapidly — from TB.
With ordinary TB, patients start to feel better after a few weeks or months on a selection of four mainstay antibiotics. But of the 542 people with TB at the hospital in 2005 and early 2006, 221 (41%) had a multi-drug-resistant (MDR) form, against which these therapies are mostly powerless. Worse, 53 of them did not even respond to the few antibiotics that form a second line of defence. Eventually, doctors had nothing left to try: all but one of the 53 died, half of them within 16 days of diagnosis. It was the first major outbreak of what became known as extensively drug-resistant (XDR) TB — and a wake-up call to the world that TB had taken a turn for the worse1.
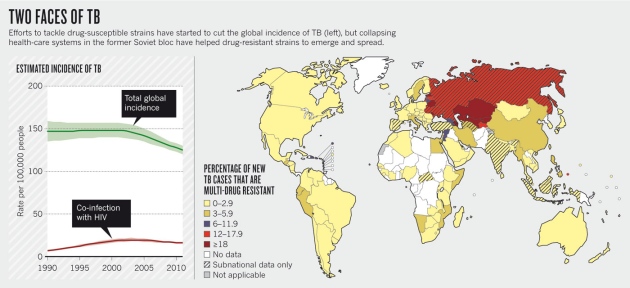
It is a tale of two TBs. Once detected, drug-sensitive TB is almost always treatable, as long as the appropriate drugs are provided and taken. Simple practices — such as checking that patients take their medicine — can be transformative. But in some countries, particularly in eastern Europe, Asia and Africa, the weakening or collapse of health-care systems over the past two decades has meant that patients do not always finish their drugs, or they take the wrong ones, allowing highly transmissible, drug-resistant strains to emerge and spread.In the early 1980s, TB cases had dropped to such low rates that Western policy-makers frequently talked of eradication of the disease. Then came the HIV epidemic, which triggered a resurgence of TB in the late 1990s. But the latest report on TB from the World Health Organization (WHO), published in October, revealed signs of progress against normal — or drug-sensitive — cases of the bacterial disease. New infections have fallen and the mortality rate has dropped by 41% since 1990. But, the report warned, “drug-resistant TB threatens global TB control”. Some 3.7% of new cases and 20% of previously treated cases are MDR-TB. And whereas in 2000 the highest incidence of MDR-TB was 14%, in Estonia; in 2010 that figure had jumped to 35%, in Russia's Arkhangelsk province. An estimated 9% of drug-resistant cases are XDR-TB, which has now been reported in 84 countries.
Drug-resistant TB is harder, more expensive and more time-consuming to treat. New tools are needed — but there have been no new anti-TB drugs in more than 50 years, and the current vaccine is largely ineffective. The most common diagnostic technique — analysing sputum samples under a microscope — can determine that Mycobacterium tuberculosis bacteria are present but not whether they are drug resistant. Meanwhile, researchers have lacked interest in developing drugs and tests, and drug companies have lacked market incentives to do so.
The growth of multi-drug resistance is an “escalating public-health emergency”, says Grania Brigden, TB adviser for Médecins Sans Frontières (Doctors Without Borders) in Geneva, Switzerland: “With barely 1 in 20 TB patients being tested for drug resistance, we're just seeing the tip of the iceberg.”
But scientists are careful to temper their alarm. In the past decade, researchers and policy-makers have fought for and won a reversal in funding and attention for TB. Several new drugs are in development, and progress is being made towards an effective vaccine.
“I do worry when people stand up at conferences and talk about MDR-TB and say it's a big disaster and the whole world is going to collapse. It's not that severe yet,” says Tim McHugh, head of the Centre for Clinical Microbiology at University College London, who leads a team that is trialling one of the two most advanced candidates for new TB drugs. “The big anxiety is that if we don't act now, it will easily run away from us.”
Rise and fall
TB is one of the world's leading killers, stealing 1.4 million lives and causing 8.7 million new and relapse infections in 2011. One-third of the world's population carries the bacterium, but most will never develop the active form of the disease.
The first modern TB epidemic took off in the late 1700s, during the Industrial Revolution. Rural workers in Europe and North America moved in droves to cities, where poverty — and related malnutrition and overcrowding — created an ideal environment for the disease's spread. But as hygiene, nutrition and medicine improved, what was known as the Great White Plague began to ebb.
“With barely 1 in 20 patients with TB being tested for drug resistance, we're just seeing the tip of the iceberg”
“By the 1940 and 50s, things looked quite bright,” says McHugh, who seems almost as interested in the history of TB as in its microbiology. The Bacillus Calmette–Guérin (BCG) vaccine, first used in the 1920s, helped. But BCG is now effective mainly against childhood TB, which is not infectious, rather than the adult form. What really broke TB's back was the introduction of isoniazid, in 1952, and then rifampicin, in the 1970s. “If you look at a graph of TB from the 1950s onward, [infection rates] just collapsed,” McHugh says.
Then, in the 1980s and 1990s, HIV hit. “You can't underestimate the importance of HIV,” McHugh says. A co-infection of TB and HIV produces a powerful biological synergy, accelerating the breakdown of the body's immune defences; latent TB infection is 20–30 times more likely to become active in people who have HIV. In 1993, the WHO declared TB a global emergency. Worldwide, TB is now the leading cause of death among people with HIV.
The resurgence of ordinary TB set the stage for drug-resistant forms. Resistance develops when people do not stick to their drug regimens — which typically last six months for drug-sensitive TB and 20 months for MDR-TB — allowing naturally occurring resistant mutants to grow and evolve. MDR-TB, which grew more threatening during the 1990s, is resistant to isoniazid and rifampicin. People with this form require second-line drugs — broad-spectrum antibiotics called fluoroquinolones or injectable agents (amikacin, capreomycin and kanamycin). These treatments are less effective, more toxic and take many more months to work than first-line therapies. An infection is classified as XDR-TB if it is also resistant to fluoroquinolones and at least one of the injectables. The XDR-TB outbreak at Tugela Ferry, when it was reported in 2006, rattled the TB research and policy community.
Experts agree that the biggest driver for the growth in drug-resistant TB has been the deterioration in some countries' health-care infrastructures, including TB programmes, since the 1990s — particularly in the former Soviet bloc. This decline has meant that patients are not diagnosed and treated; and in some countries, over-the-counter availability of anti-TB drugs also encourages people to take inappropriate second-line therapies, accelerating the growth of drug resistance. In a stroke of bad luck, the virulent and often drug-resistant 'Beijing' strain of TB, identified in 1995 in China, swept through Russia and eastern Europe just as the region's public-health provision was being dismantled. “There was a confluence of the biology of the organism and its progress into Russia, where lots of people had a health-care system that was collapsing around their ears,” says McHugh. (In 2010, the Beijing strain was found in around 13% of active TB infections worldwide.) All of this helps to explain why the recent WHO report shows the highest burden of MDR TB in Russia's Arkhangelsk province and in Belarus, Estonia, Kazakhstan, Kirghizia and Moldova (see 'Two faces of TB').
Fighting back
Over the past decade or so, the paths of drug-sensitive and drug-resistant TBs have diverged. The solution for drug-sensitive TB is simply to deliver the drugs and diagnostics to patients — and the drive to do so has grown. One of the United Nations Millennium Development Goals set in 2000 was to halt and begin to reverse the incidence of TB by 2015; in 2001, the international Stop TB Partnership was established, bringing together government programmes, researchers, charitable foundations, non-governmental organizations (NGOs) and the private sector.
One major result of these and other efforts was a global expansion of 'directly observed treatment, short course' (DOTS), a strategy promoted by the WHO to combat drug-sensitive TB. Once diagnosed, the disease is treated with a supply of first-line drugs, taken under the close observation of health-care workers to ensure that people finish the course. Thanks in large part to such efforts, the WHO says that the world is on track to halve TB mortality from 1990 levels by 2015.
Tackling drug-resistant TB, however, will require not just the rebuilding of health-care infrastructure, but also new weapons, such as diagnostics, drugs and vaccines. The private sector has had little incentive to invest in basic research whose eventual products, if any emerge, would largely be sold at low cost in poor countries. “They have to look at the bottom line,” says Anthony Fauci, head of the US National Institute of Allergy and Infectious Diseases in Bethesda, Maryland.
In the past decade or so, global TB programmes have pumped money into research. One major development came in 1998, when researchers at the Wellcome Trust Sanger Institute in Hinxton, UK, published the genome sequence of M. tuberculosis, allowing researchers to identify and study genes that underlie the bacterium's virulence and ability to evade the immune system2. In 2012, the US National Institutes of Health in Bethesda started a bigger genome-sequencing project that aims to uncover the genetic roots of drug resistance. “We'll use next-generation sequencing technologies to sequence 1,000 TB clinical isolates from around the world — South Africa, Korea, Russia, Uganda — anywhere drug-resistant TB is heavily present,” says Fauci.
War chest
There are now ten TB drugs in clinical trials. The aim is to find compounds that are effective against resistant strains and that work faster and have fewer side effects, so that patients will be more likely to finish the course. McHugh and his team, for example, are running a clinical trial at sites across Africa and Asia to test the antibiotic moxifloxacin, which is commonly used for pneumonia and skin infections. (They expect to release preliminary results in 2013.) The researchers are also working to speed up the screening process for potential drugs by using mycobacterial species that are less pathogenic and more fecund than M. tuberculosis, which is slow-growing, finicky and poses a biosecurity risk. “Previously, what you had was a chemist who says 'I've got this molecule that will killEscherichia coli. I'm fairly sure it should kill TB. But there's nowhere I can see if it does,'” McHugh says.
Accurate and fast diagnostic tests for drug-resistant strains are also a key part of the fight, and a number of tests have come online in the past five years. One, called GeneXpert, takes 90 minutes to complete and is based on a gene-amplification technique that detects DNA sequences specific to M. tuberculosis and to rifampicin resistance. The system has been endorsed by the WHO and subsidized by a coalition of organizations, but researchers are still seeking simpler, cheaper options.
Only better vaccines will solve the problem for good. “We must invest in vaccine research if our ultimate goal is to be able to prevent the disease rather than forever chase growing drug resistance,” says Helen McShane, a vaccine researcher at the University of Oxford, UK.
“We must invest in vaccine research if our ultimate goal is to be able to prevent the disease.”
In 2008, the European Commission pushed for the creation of the TB Vaccine Initiative, which draws funding from European countries, NGOs and private funders. These and other efforts have helped to boost the number of vaccine candidates from 0 to 12 since 2000.
McShane and her team are on the cusp of the first efficacy results for MVA85A, one of the most clinically advanced TB vaccines in the pipeline at present. The shot, which McShane helped to develop as a PhD student 15 years ago, contains a virus designed to ramp up the activity of T cells that have already been primed by BCG. In 2009, in partnership with the South African Tuberculosis Vaccine Initiative, McShane launched a major phase II clinical trial on nearly 3,000 BCG-vaccinated babies in South Africa; early results are expected in the first quarter of 2013. In parallel, she and her colleagues are also testing the vaccine's efficacy in HIV-infected adults in South Africa and Senegal.
But are these efforts enough? “Unfortunately not,” concludes Karin Weyer, coordinator of laboratories, diagnostics and drug resistance at the WHO Stop TB Department in Geneva. Annual funding for TB diagnosis and treatment is expected to reach some US$4.8 billion in 2013 — but TB care and control are expected to demand up to $8 billion a year by 2015. The $600 million contributed to TB research in 2010 also falls well short of the $2 billion the WHO estimates will be needed annually — and the economic crisis has slowed financing across the board. “I need to be and want to be optimistic,” says Weyer. “But we're still working with shoestring budgets compared to HIV.”
Meanwhile, the bacterium is not resting. In December last year, clinicians in Mumbai, India, reported3the identification of 12 patients with what they termed totally drug-resistant TB, or TDR-TB. Similar claims had been made a few years earlier in Italy and Iran, but this time the WHO took it seriously enough to investigate. In March 2012, 40 experts convened by the WHO concluded that there was not enough evidence to say that TDR-TB was substantially different from XDR-TB.
McHugh agrees. But he does not need further evidence to act. In the face of marching drug resistance, it is the responsibility of researchers to speak out, he says. “I think we can no longer be scientists in our labs doing fascinating stuff and think we're doing good work. We have to evangelize a little bit too.”
- Nature
- 493,
- 14–16
- ()
- doi:10.1038/493014a











No comments:
Post a Comment