17 DEC, 2012
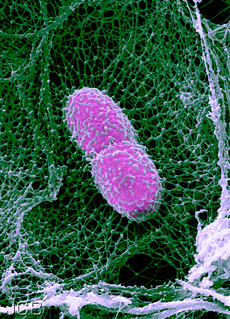
A scanning electron micrograph of an infected mouse lung shows a Klebsiella pneumoniae bacterium (pink) snared in a neutrophil extracellular trap (green), a web of decondensed chromatin released by neutrophils to catch and kill pathogens.
Catherine Carver meets the unsung super soldiers in each and every one of us.
I’d like to tell you a secret. I am a superhero. I can devour my enemies whole, release my own chemical weapons and trap and kill my prey in nets spun from my own DNA. And I don’t even need to wear my pants on the outside. I am a neutrophil, and several billion of me are made in your bone marrow every day.
Neutrophils are the most abundant white blood cell in the human body. They play a vital role in an ancient, rapid response called the innate immune system – the first line of defence against disease-causing microbes. This system can mount a protective offence within minutes of the body being invaded, before the nature of the attack is known, buying time for it to produce a more tailored response.
The neutrophil is at the heart of the action, a killing machine that destroys unwanted intruders. It has many enemies. Whether a snot-filled toddler, a slobbery dog or a propensity for paper cuts, there will be something that exposes you to infection. Within minutes of infection invading your body the damaged tissue releases a chemical distress signal, attracting neutrophils out of the bloodstream, battle ready.
In their activated state neutrophils have three key strategies. First, they can engulf and devour microbes. This process, phagocytosis, was first described over one hundred years ago by Ilya Mechnikov who won the Nobel Prize for Medicine for the discovery in 1908. The process is directed by molecular tags called opsonins, which stick to microbes. If the microbe were a cookie, opsonins would be a bit like chocolate chips, making it all the more appealing to the hungry neutrophil.
The second strategy is to kill microbes in the local area. Neutrophils do this by releasing packets of enzymes, which attack the outside of the microbe. This is a bit like pouring boiling oil on the invaders: crude but effective, though it can cause collateral damage to the very tissue the neutrophils are meant to protect….
Any damage is limited though because neutrophils are also fearless kamikaze warriors of a sort. They die 24-48 hours after moving into the tissue. You can see this as the bodies accumulate on the battlefield as pus. Yet even in death they have one final weapon: NETs.
NETs (Neutrophil Extracellular Traps) are created once a neutrophil’s self-destruct programme has been activated. DNA, proteins and hostile enzymes mingle until a neutrophil bursts open in a final sacrificial act, unleashing a web that traps and kills bacteria. This works against an array of different bacteria, from Shigella, which causes dysentery, to Salmonella, which is responsible for typhoid fever.
Understanding this triad of killer skills is an important area of biomedical research. Neutrophils are designed to be part of a hard-and-fast response. If the battle is drawn out longer than expected or the neutrophils attack too enthusiastically it can cause more harm than good. Campaigns have gone awry leading to autoimmune diseases such as rheumatoid arthritis and emphysema. By understanding how neutrophils cause damage scientists hope to design new anti-inflammatory drugs to help tone down the response and tackle these crippling conditions.
But don’t forget: you need neutrophils. Without their skills you couldn’t go for a stroll in a park or kiss someone without risking death by infection. This is a reality for people on certain chemotherapy regimes, which decimate neutrophil numbers. You hear a lot about the enemy – superbugs, anthrax, flesh-eating bacteria. You’ve celebrated pharmacological victories like penicillin. It’s time to recognise the remarkable feats happening inside each and every one of you every day.
Catherine Carver
Catherine Carver is a Grants Advisor at the Wellcome Trust and blogs at A Little Grey Cell. This is an edited version of Catherine’s original essay. Views expressed are the author’s own.
This essay was originally shortlisted for the 2012 Wellcome Trust Science Writing Prize, but withdrawn due to a sudden conflict of interest (Catherine got a job with us!). We still love the essay though, which has also been published on Scientific American’s guest blog.
Find out more about the Wellcome Trust Science Writing Prize in association with the Guardian and the Observer and read our ‘How I write about science‘ series of tips for aspiring science writers.
Read the other shortlisted essays from the 2012 and 2011 competitions in our archive.











No comments:
Post a Comment